- News
- Education News
- Learning with TOI News
- Atomic structure explained: The building blocks of matter
Trending
This story is from June 7, 2023
Atomic structure explained: The building blocks of matter
Atoms are the fundamental components of matter, made up of three subatomic particles: protons, neutrons, and electrons. The structure of atoms determines the properties and behavior of different substances.
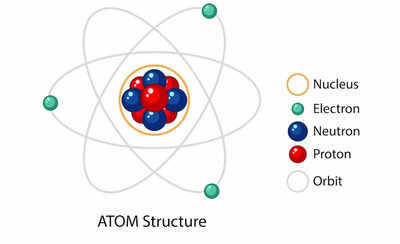
Atom structure (Image source: Freepik)
Atoms are the basic building blocks of matter. They are incredibly tiny particles that make up everything around us, from the air we breathe to the objects we interact with. Understanding the structure of atoms is key to understanding the properties and behavior of different substances.
Atoms consist of three main subatomic particles: protons, neutrons, and electrons.
For example, Sodium has 11 electrons, with 2 in the first shell and 8 in the second shell. The outermost shell contains 1 electron, which makes sodium highly reactive and likely to form chemical bonds.
Understanding the
(The content is generated with the assistance of Artificial Intelligence)
Protons, Neutrons, and Electrons: The Three Subatomic Particles
Atoms consist of three main subatomic particles: protons, neutrons, and electrons.
- Protons: Protons are positively charged particles found in the nucleus (central core) of an atom. They have a relative mass of 1 and a positive charge of +1.
- Neutrons: Neutrons are neutral particles also located in the nucleus of an atom. They have a relative mass of 1, just like protons, but carry no charge (neutral).
- Electrons: Electrons are negatively charged particles that orbit around the nucleus in regions called electron shells or energy levels. They have a very small mass compared to protons and neutrons.
For example, Carbon atoms have 6 protons, 6 neutrons, and 6 electrons. The protons and neutrons are tightly packed in the nucleus at the center, while the electrons move around the nucleus in specific energy levels.
Atomic Number and Atomic Mass
- Atomic Number: The atomic number of an atom is determined by the number of protons it has. It uniquely identifies each element on the periodic table. For example, Oxygen has an atomic number of 8 because it has 8 protons in its nucleus.
- Atomic Mass: The atomic mass of an atom is the sum of the protons and neutrons in its nucleus. It is expressed in atomic mass units (amu). For example, Carbon-12, the most common form of carbon, has an atomic mass of 12 amu, which includes 6 protons and 6 neutrons.
Electron Shells and Valence Electrons
- Electron Shells: Electrons occupy specific energy levels or electron shells around the nucleus. The innermost shell can hold up to 2 electrons, while the subsequent shells can hold more.
- Valence
Electrons : The electrons in the outermost shell of an atom are called valence electrons. They are crucial for the chemical behavior of atoms, as they determine how atoms interact and form chemical bonds.
For example, Sodium has 11 electrons, with 2 in the first shell and 8 in the second shell. The outermost shell contains 1 electron, which makes sodium highly reactive and likely to form chemical bonds.
Understanding the
atomic structure helps explain why elements have different properties and behaviors. It allows us to identify elements based on their atomic number, determine their reactivity based on the number of valence electrons, and understand how atoms come together to form molecules through chemical bonding.
(The content is generated with the assistance of Artificial Intelligence)
End of Article
FOLLOW US ON SOCIAL MEDIA










