1.3 Atomic Structure - Chemistry Teaching Resources
1.3 Atomic Structure - Chemistry Teaching Resources
1.3 Atomic Structure - Chemistry Teaching Resources
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chemical Changes & <strong>Structure</strong> 16/06/2013<br />
<strong>1.3</strong> <strong>Atomic</strong> <strong>Structure</strong><br />
This lesson topic revises and extends your understanding of <strong>Atomic</strong> <strong>Structure</strong>.<br />
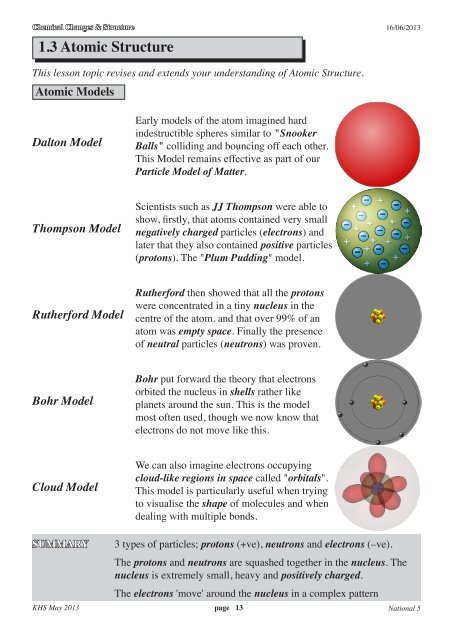
<strong>Atomic</strong> Models<br />
Dalton Model<br />
Thompson Model<br />
Rutherford Model<br />
Bohr Model<br />
Cloud Model<br />
Early models of the atom imagined hard<br />
indestructible spheres similar to "Snooker<br />
Balls" colliding and bouncing off each other.<br />
This Model remains effective as part of our<br />
Particle Model of Matter.<br />
Scientists such as JJ Thompson were able to<br />
show, firstly, that atoms contained very small<br />
negatively charged particles (electrons) and<br />
later that they also contained positive particles<br />
(protons). The "Plum Pudding" model.<br />
Rutherford then showed that all the protons<br />
were concentrated in a tiny nucleus in the<br />
centre of the atom. and that over 99% of an<br />
atom was empty space. Finally the presence<br />
of neutral particles (neutrons) was proven.<br />
Bohr put forward the theory that electrons<br />
orbited the nucleus in shells rather like<br />
planets around the sun. This is the model<br />
most often used, though we now know that<br />
electrons do not move like this.<br />
We can also imagine electrons occupying<br />
cloud-like regions in space called "orbitals".<br />
This model is particularly useful when trying<br />
to visualise the shape of molecules and when<br />
dealing with multiple bonds.<br />
SUMMARY 3 types of particles; protons (+ve), neutrons and electrons (–ve).<br />
The protons and neutrons are squashed together in the nucleus. The<br />
nucleus is extremely small, heavy and positively charged.<br />
The electrons 'move' around the nucleus in a complex pattern<br />
KHS May 2013 page 13<br />
National 5
Chemical Changes & <strong>Structure</strong> 16/06/2013<br />
Important Numbers<br />
<strong>Atomic</strong> Number - is<br />
the number of protons<br />
in the nucleus of all<br />
atoms in an element<br />
Mass Number - is<br />
the total number of<br />
protons and neutrons<br />
in the nucleus of an<br />
atom<br />
Electrons - In neutral atoms the number of electrons is equal to the<br />
number of protons so we can usually use the <strong>Atomic</strong> Number<br />
to tell us the number of electrons as well.<br />
Neutrons - The number of neutrons is simply the number of protons<br />
(<strong>Atomic</strong> Number) subtracted from the Mass Number.<br />
KHS May 2013 page 14<br />
Each element has a different atomic number and<br />
they are listed in order of this number. Elements with<br />
similar properties are found in the same group.<br />
National 5
Chemical Changes & <strong>Structure</strong> 16/06/2013<br />
Element Symbol <strong>Atomic</strong> Mass number of number of number of<br />
Number Number protons electrons neutrons<br />
Nitrogen<br />
Oxygen<br />
Neon<br />
Sodium<br />
Magnesium<br />
Silicon<br />
Phosphorus<br />
Sulphur<br />
Potassium<br />
Nickel<br />
Zinc<br />
Silver<br />
Tin<br />
Platinum<br />
Mercury<br />
N 7 14 7 7 14 - 7 = 7<br />
O<br />
Ne<br />
Na<br />
Mg<br />
Si<br />
P<br />
S<br />
K<br />
Ni<br />
Zn<br />
Ag<br />
Sn<br />
Pt<br />
Hg<br />
KHS May 2013 page 15<br />
8<br />
10<br />
11<br />
12<br />
14<br />
15<br />
16<br />
19<br />
28<br />
30<br />
47<br />
50<br />
84<br />
80<br />
16<br />
20<br />
23<br />
24<br />
28<br />
31<br />
32<br />
39<br />
59<br />
66<br />
108<br />
119<br />
195<br />
201<br />
Number of protons = <strong>Atomic</strong> Number<br />
8<br />
10<br />
11<br />
12<br />
14<br />
15<br />
16<br />
19<br />
28<br />
30<br />
47<br />
50<br />
84<br />
80<br />
8<br />
10<br />
11<br />
12<br />
14<br />
15<br />
16<br />
19<br />
28<br />
30<br />
47<br />
50<br />
84<br />
80<br />
66 - 30 = 36<br />
Number of electrons = Number of protons = <strong>Atomic</strong> Number<br />
Number of neutrons = Total in nucleus - Number of protons<br />
= Mass Number - <strong>Atomic</strong> Number<br />
The Mass Number can only ever refer to one particular atom. However, when<br />
we want to talk generally about the mass of the atoms of an element, we can<br />
usually safely assume that the average mass (RAM) rounded to the nearest<br />
whole number can safely be used as the 'most likely' Mass Number for an atom<br />
of this element - but be careful, Hg has RAM 79.9 so we would assume 'most<br />
likely' Mass Number = 80, but only 79 Hg and 81 Hg exist naturally.<br />
National 5
Chemical Changes & <strong>Structure</strong> 16/06/2013<br />
Nuclide Notation Nuclide Notation is the system which adds information<br />
about an atom to its Symbol.<br />
proton in the nucleus 1<br />
Isotopes<br />
2<br />
1 H<br />
Heavy hydrogen<br />
(deuterium)<br />
1<br />
1 H<br />
Ordinary hydrogen<br />
3<br />
1 H<br />
Very heavy hydrogen<br />
(tritium)<br />
Relative <strong>Atomic</strong> Mass (RAM)<br />
KHS May 2013 page 16<br />
⎯⎯→<br />
⎯⎯→<br />
1 0<br />
orbiting around the nucleus - 1<br />
mass number<br />
atomic number<br />
7<br />
3 Li<br />
Isotopes are atoms of the same element which<br />
have the same number of protons but have<br />
different numbers of neutrons.<br />
This means that atoms of the same element<br />
can have different masses .<br />
Isotopes are atoms of the same atomic number but<br />
different mass numbers .<br />
Since atoms of the same element can<br />
have different masses, it is necessary to<br />
know the average mass - the<br />
relative atomic mass of an element.<br />
Information provided by a machine<br />
called a mass spectrometer can be used<br />
to calculate the RAM of an element.<br />
National 5
Chemical Changes & <strong>Structure</strong> 16/06/2013<br />
① Each atom has an electron knocked<br />
off which leaves the atom as a<br />
positively charged ion .<br />
①<br />
➁ The ions are accelerated by an electric field; repelled<br />
by a positive plate, attracted towards a negative.<br />
➂ The strength of the magnetic field is gradually increased.<br />
④ Any ions that are of the correct mass will be deflected ‘round the corner’.<br />
⑤ Any ions which are still too heavy for the magnetic field will crash into<br />
the wall of the chamber. They will be detected later when the field is<br />
stronger.<br />
⑥➆ Any ions which are too light will be deflected too far. They would have<br />
been detected earlier when the field was weaker.<br />
⑦ Any ions arriving here are detected and counted.<br />
The mass spectrometer is able to tell us 3 things about an element:<br />
1. the number of isotopes that element has,<br />
2. the mass number of each isotope, and<br />
3. the relative amounts of each isotope.<br />
The information is printed out in the form of a mass spectrum.<br />
KHS May 2013 page 17<br />
➁<br />
④<br />
➂<br />
⑥<br />
⑦<br />
⑤<br />
National 5
Chemical Changes & <strong>Structure</strong> 16/06/2013<br />
<strong>Atomic</strong><br />
No. (Z)<br />
3 Lithium<br />
5 Boron<br />
12 Magnesium<br />
14 Silicon<br />
24 Chromium<br />
* Some values within this table have been rounded / modified for simplicity<br />
KHS May 2013 page 18<br />
From this information it is now possible to<br />
calculate the average mass of all the atoms<br />
in the element.<br />
RAM = ( mass 1 x % 1 ) + ( mass 2 x % 2 )<br />
___________________________<br />
100<br />
= ( 35 x 75 ) + ( 37 x 25 )<br />
___________________________<br />
100<br />
= ( 3575 ) + ( 37 )<br />
___________________________<br />
100<br />
=<br />
Name Symbol % Abundance RAM (Relative <strong>Atomic</strong> Mass)<br />
6 Li<br />
7 Li<br />
10 B<br />
11 B<br />
24 Mg<br />
25 Mg<br />
26 Mg<br />
28 Si<br />
29 Si<br />
30 Si<br />
50 Cr<br />
52 Cr<br />
53 Cr<br />
54 Cr<br />
7.59<br />
92.41<br />
19.90<br />
80.10<br />
78.99<br />
10.00<br />
11.01<br />
92.23<br />
4.68<br />
3.09<br />
4.35<br />
83.79<br />
9.50<br />
2.36<br />
National 5
Chemical Changes & <strong>Structure</strong> 16/06/2013<br />
Isotopic Ions<br />
It is not just the number of neutrons that can be different<br />
in atoms of the same element. Atoms can also change their<br />
number of electrons.<br />
7<br />
3 Li 6<br />
3 Li 7<br />
3 Li+ 6<br />
3 Li+<br />
protons = 3<br />
neutrons = 4<br />
electrons = 3<br />
protons = 3<br />
neutrons = 3<br />
electrons = 3<br />
KHS May 2013 page 19<br />
protons = 3<br />
neutrons = 4<br />
electrons = 2<br />
protons = 3<br />
neutrons = 3<br />
electrons = 2<br />
The number of protons never changes. This is why the <strong>Atomic</strong> Number for an<br />
element is defined as the number of protons.<br />
Element Symbol <strong>Atomic</strong> Mass number of number of number of<br />
Number Number protons neutrons electrons<br />
Lithium<br />
Oxygen<br />
Chlorine<br />
Sodium<br />
Phosphorus<br />
Iron (II)<br />
Iron (III)<br />
Tin (II)<br />
Tin (IV)<br />
7 + Li 3<br />
16<br />
8<br />
O 2-<br />
37<br />
- Cl 17<br />
23<br />
+ Na 11<br />
31<br />
3- P 15<br />
56<br />
2+ Fe 26<br />
58<br />
3+ Fe 26<br />
2 + H 1<br />
116<br />
2+ Sn 50<br />
119<br />
4+<br />
Sn 50<br />
3<br />
8<br />
17<br />
11<br />
15 31 15 15 16<br />
26<br />
26<br />
1<br />
50<br />
50<br />
7<br />
16<br />
37<br />
23<br />
56<br />
58<br />
2<br />
116<br />
119<br />
3<br />
8<br />
17<br />
11<br />
26<br />
26<br />
1<br />
50<br />
50<br />
4<br />
8<br />
20<br />
12<br />
30<br />
32<br />
1<br />
66<br />
69<br />
2<br />
10<br />
18<br />
10<br />
18<br />
24<br />
23<br />
0<br />
48<br />
46<br />
National 5
Chemical Changes & <strong>Structure</strong> 16/06/2013<br />
Q1. SC Q5. Int2<br />
The grid shows information about some particles.<br />
Number of<br />
Particle protons neutrons electrons<br />
A 11 12 11<br />
B 9 10 9<br />
C 11 13 11<br />
D 19 20 18<br />
E 9 10 10<br />
a) Identify the particle which is a negative ion.<br />
_______<br />
a) Identify the two particles which are isotopes.<br />
_______ and _______<br />
Q2. Int2<br />
An atom has 26 protons, 26 electrons and 30 neutrons. The<br />
atom has.<br />
A atomic number 26, mass number 56<br />
B atomic number 26, mass number 52<br />
C atomic number 30, mass number 56<br />
D atomic number 30, mass number 82<br />
Q3. Int2<br />
Which line in the table describes a neutron?<br />
Mass Charge<br />
A 1 - 1<br />
B negligible 0<br />
C 1 + 1<br />
D 1 0<br />
Q4. Int2<br />
The isotopes of carbon and oxygen are given in the table.<br />
12<br />
Isotopes of carbon C 6<br />
16<br />
Isotopes of oxygen O 8<br />
13<br />
C 6<br />
17<br />
O 8<br />
14<br />
C 6<br />
18<br />
O 8<br />
A molecule of carbon dioxide with mass 46 could contain<br />
A one 12 C atom and two 16 O atoms<br />
B one 14 C atom and two 18 O atoms<br />
C one 12 C atom, one 16 O atoms and one 18 O atom<br />
D one 14 C atom, one 16 O atoms and one 18 O atom<br />
KHS May 2013 page 20<br />
In the manufacture of glass, other chemicals can be added<br />
to alter the properties of the glass. The element boron can<br />
be added to glass to make oven proof dishes.<br />
Information about an atom of boron is given below.<br />
Particle Number<br />
proton 5<br />
electron 5<br />
neutron 6<br />
Use this information to complete the nuclide notation for<br />
this atom of boron.<br />
B<br />
Atoms of boron exist which have the same number of protons<br />
but a different number of neutrons from that shown<br />
in the table.<br />
What name can be used to describe the different atoms of<br />
boron?<br />
______________________________________<br />
Q6. Int2<br />
Elements are made up of atoms.<br />
An atom of an element is represented by the diagram<br />
below.<br />
= protons<br />
= neutrons<br />
= electrons<br />
What name is given to the part of the atom which contains<br />
protons and neutrons?<br />
______________________________________________<br />
Using the information in the diagram:<br />
a) state the mass of this atom;<br />
______________________<br />
b) explain why this atom is electrically neutral;<br />
_______________________________________<br />
_______________________________________<br />
c) name the family of elements to which this atom<br />
belongs.<br />
_______________________________________<br />
National 5